Microbial Electrochemical Systems
Microbial electrochemical technologies exploit electro-active microorganisms to recover energy or valuable products while purifying wastewater.
Microbial electrochemical technologies exploit electro-active microorganisms to recover energy or valuable products while purifying wastewater.
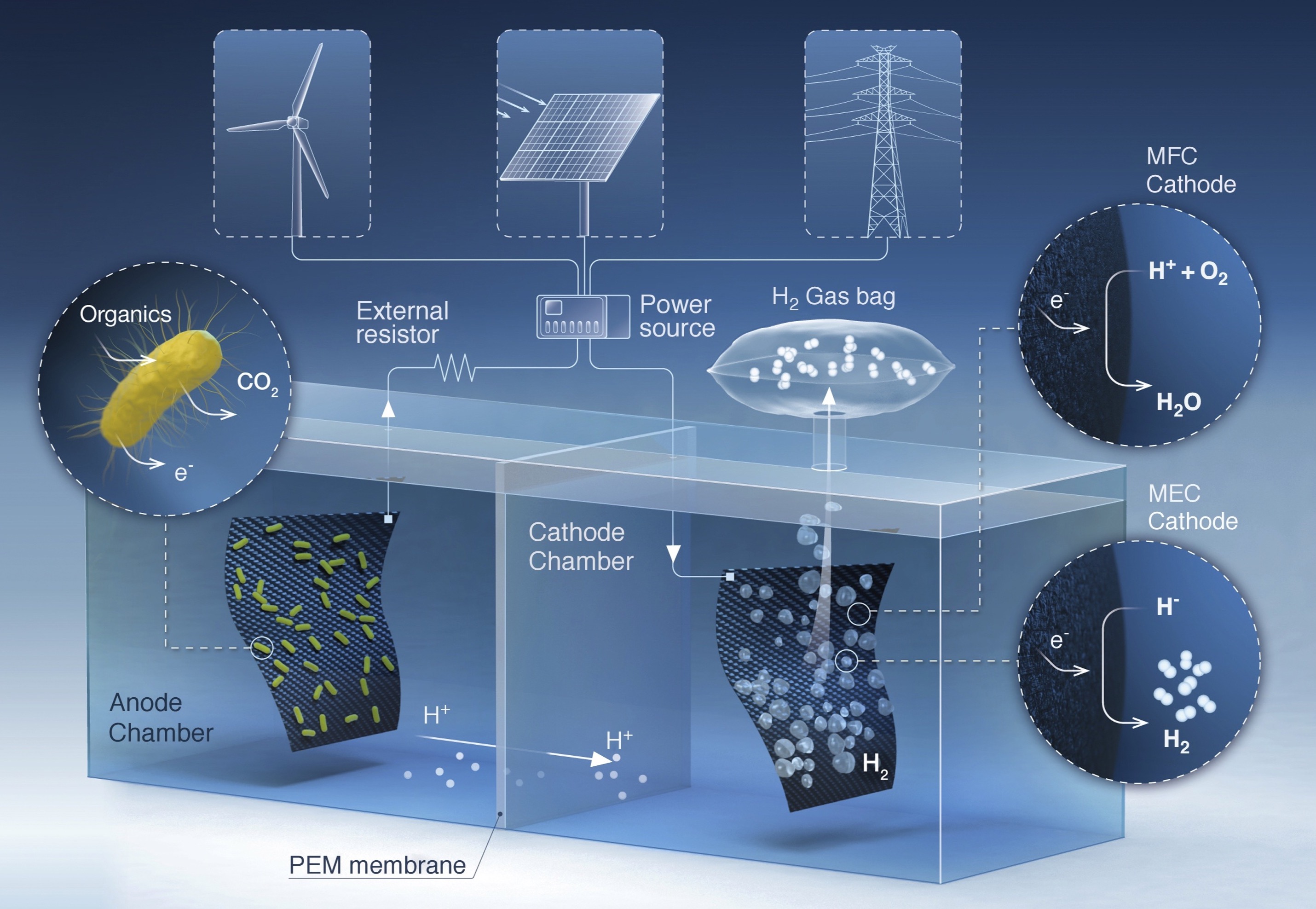
In a microbial electrolysis cell, biofilms growing on the anode oxidize the organics in wastewater and release electrons. A small voltage - typically sourced from photovoltaics or a battery - drives those electrons to a cathode, where hydrogen gas forms. The approach cleans the water and captures a usable fuel with minimal aeration energy.
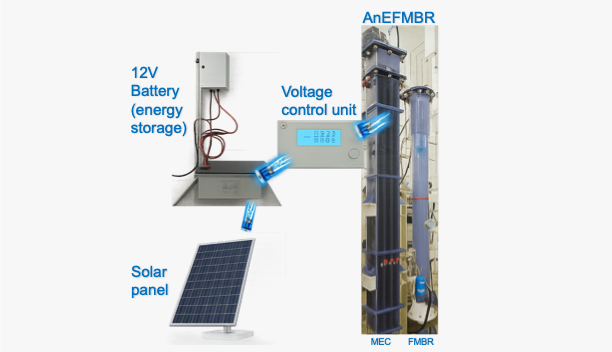
Secured by international patent US10059609B2, our first-of-its-kind anaerobic electrochemical fluidized membrane bioreactor (AnEFMBR) blends MEC chemistry with membrane filtration. Granular activated carbon (GAC) grains stay gently fluidized around hollow-fiber ultrafiltration membranes, acting both as high-surface-area electrodes for electro-active microbes and as microscopic scrubbers that keep the fibers clean. The system converts municipal wastewater into reusable water and simultaneously recovers methane-rich biogas, achieving a net-positive energy balance and meeting non-potable reuse standards.

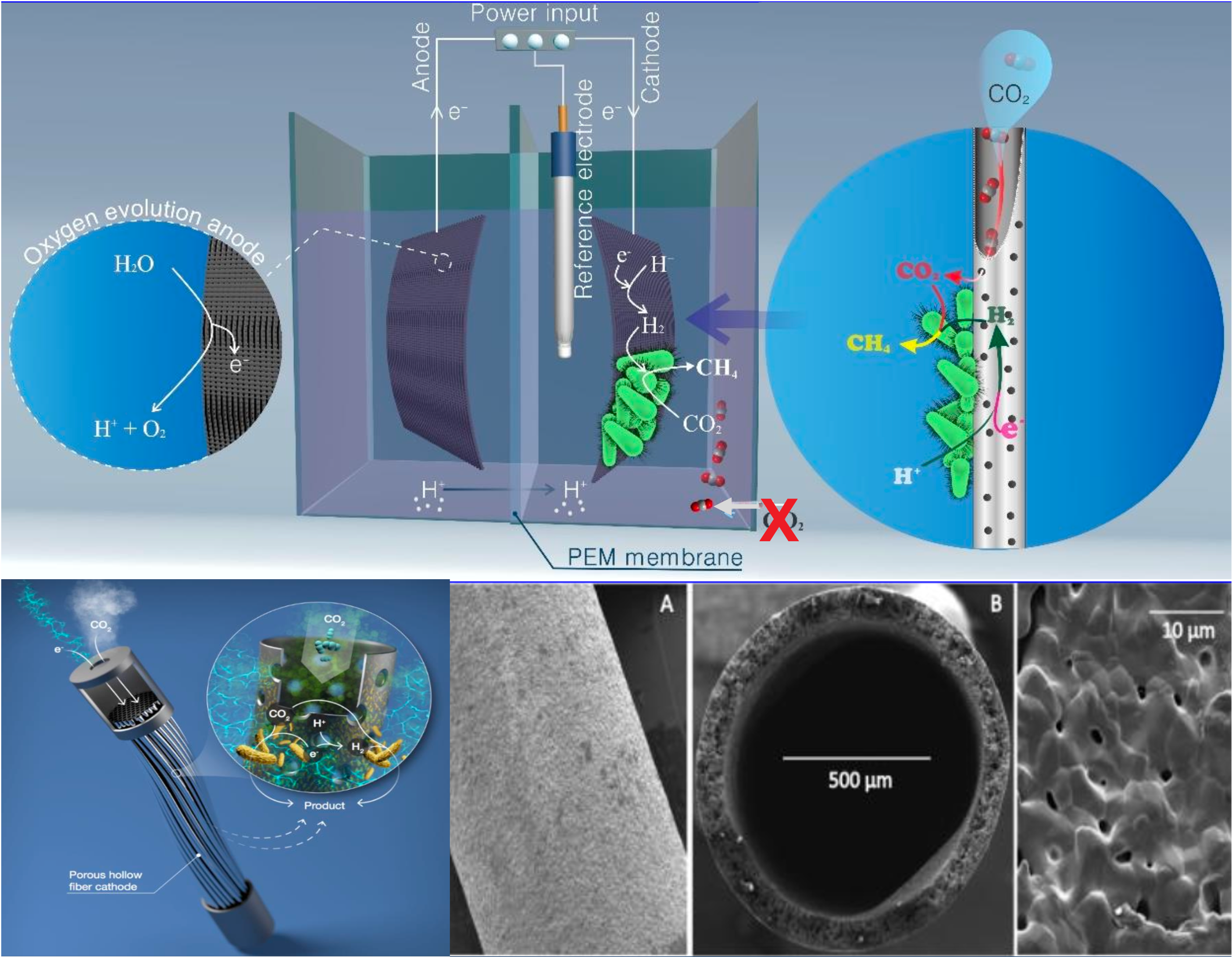
Microbial electrosynthesis (MES) is a bioelectrochemical system that utilizes microorganisms as biocatalysts to convert CO₂ into high-value products. In a MES process, hemilithoautotrophic bacteria uptake electrons directly or indirectly (e.g., as H₂ or formate) from a cathode to reduce CO₂ into fuels or high-value chemicals like acetate and ethanol at low potentials. Since MES uses electricity as the energy source, it can be powered by wind or solar, offering a clean, carbon-neutral path to sustainable fuels and chemicals.
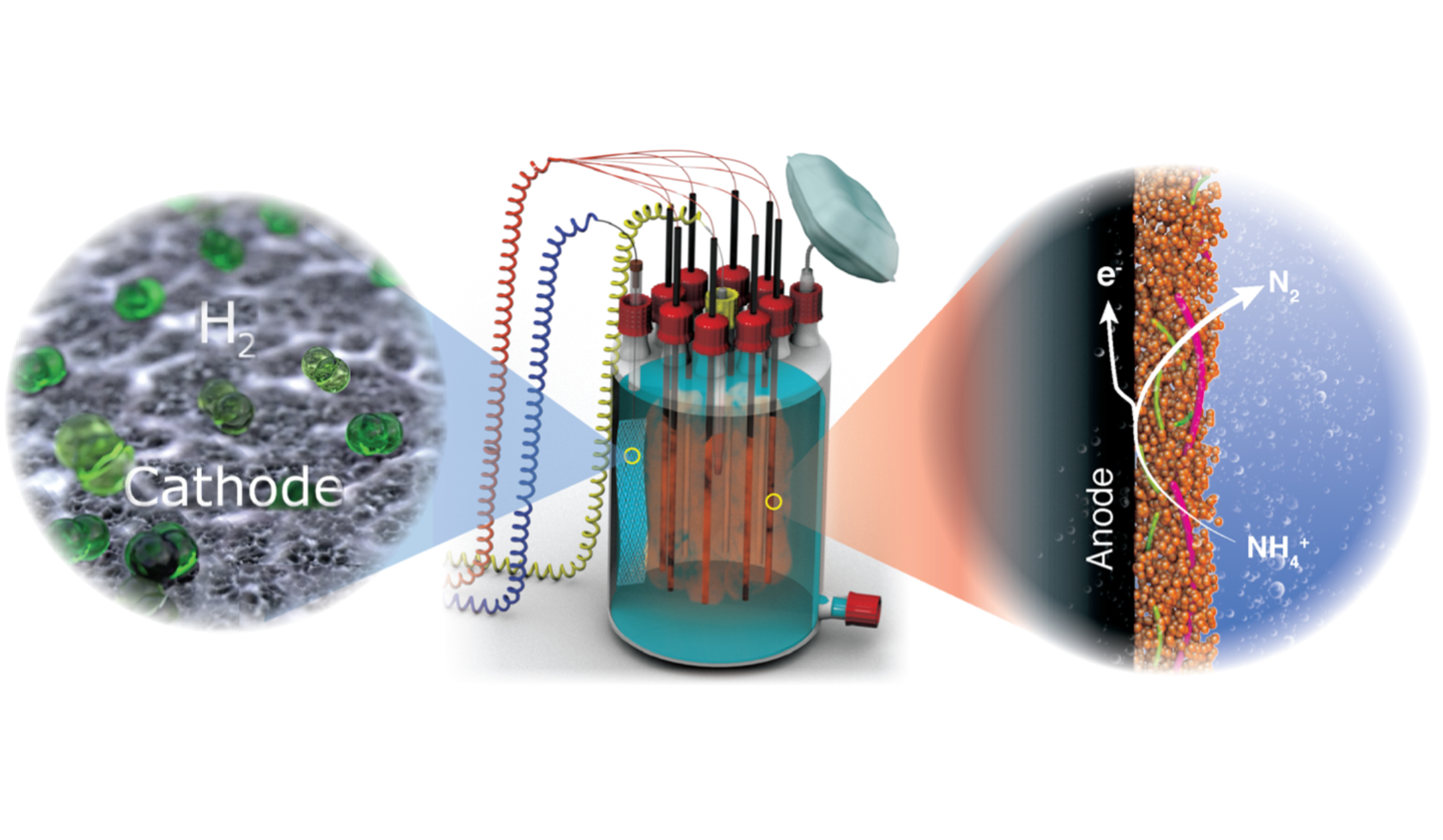
The anaerobic ammonium oxidation (anammox) process by anammox bacteria plays a significant role in achieving energy-efficient wastewater treatment (WWT). In the anammox process, ammonium (NH₄⁺) is directly oxidized to dinitrogen (N₂) gas using nitrite (NO₂⁻) as the electron acceptor.
Recently, our research group discovered that anammox bacteria are electroactive and can transfer the electrons generated from the oxidation of ammonium to the anode through extracellular electron transfer (EET). The process does not accumulate nitrite or nitrate nor produce the greenhouse gas N₂O. Additionally, the energy released from ammonium oxidation can be captured in the form of energy-rich hydrogen gas (H₂) at the cathode.